Agdia-Emea has commercialized a rapid and user-friendly DNA test kit, based on the AmplifyRP® XRT+ platform, for detection of Xylella fastidiosa (Xf).
Agdia-Emea AmplifyRP XRT+ platform works similarly to the recently launched XRT platform in that the results can be obtained using a portable fluorometer. However, XRT+ provides greater flexibility because the test can alternatively be performed using a small portable heat block with results being visualized post-amplification on an Amplicon Detection Chamber. This allows end-users who don’t want to invest in a fluorometer an alternative method of running the test.
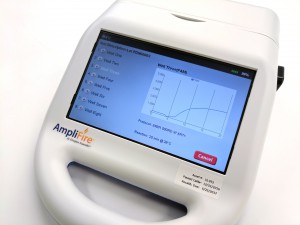
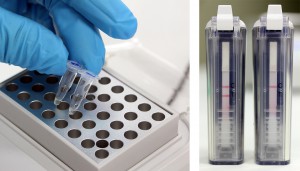
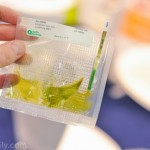
To run the test, an end-user first extracts their sample by adding it, along with extraction buffer, to a micro centrifuge tube that is provided in the kit. After the sample has been extracted a small volume is added to a solution that is used to rehydrate a lyophilized reaction pellet. Once the reaction pellet has been rehydrated it can be added to a portable fluorometer or a small heat block for 20 minutes. During this time, if present, Xf DNA will be amplified. If using the fluorometer results will automatically be displayed in real-time (photo 1). Alternatively, if using a heat block, the reaction is added to an Amplicon Detection Chamber following the amplification step where results will be visible on a lateral flow strip (photo 2).
The kit includes 24 or 48 reactions and the necessary buffers to perform the test. If performing the test as a real-time assay, the end-user will need to purchase a portable fluorometer separately. Agdia claims the test is compatible with several commercially available fluorometers but recommends the AmpliFire® (Photo 1), manufactured by Douglas Scientific. The AmpliFire is battery operated and has a small footprint, making it possible to test in remote locations.
Alternatively, if the end-user runs the test as an end-point detection assay they will need to purchase Amplicon detection chambers, as well as a starter pack, separately. The starter pack includes a heat block (necessary for the amplification step) as well as pipettes, pipette tips, and a tube rack.
The test is also offered as a service by Agdia Testing Services for those who wish to send samples for diagnosis. For more information on the test contact Agdia-Emea at +33 1 60 78 81 64 or via email at info@agdia-emea.com